Section :
Augmorix
Colour Information :
268 C
Black
- Dosage in hepatic impairment : Caution should be taken when given the dose and monitor hepatic
function at regular intervals.
RESTRICTIONS ON USE :
Contraindications
- Hypersensitivity to ß-lactams.
- Previous history of Amoxicillin - Clavulanate associated jaundice/hepatic impairment.
Precautions
- Prolong use of Augmorix may result in overgrowth of non-susceptible organisms.
- Appropriate monitoring should be taken for prothrombin time when anticoagulants are prescribed
concomitantly with Augmorix.
- Augmorix dosage should be adjusted in pateints with renal impairment .
- Augmorix should be used with caution in patients with evidence of hepatic dysfunction.
- It is advisable to maintain adequate fluid intake during the administration of high doses of Amoxicillin to
reduce the possibility of Amoxicillin crystalluria.
- Clavulanic acid may cause false positive Coombs test.
Effects on the ability to drive vehicles or use machines
Administer with caution since it may cause dizziness .
Use in Pregnancy and Lactation
Augmorix should be avoided in pregnancy especially during the first trimester, unless considered essential
use by the physician. Augmorix is excreted into breast milk, so it can be used during the period of lactation
when necessary.
DRUG INTERACTIONS :
- Augmorix may reduce the efficacy of oral contraceptives.
- Concomitant use of Augmorix with probenecid may increase and prolong blood level of Amoxicillin but
not Clavulanic Acid .
- Concomitant use of Augmorix with methotrexate may reduce the excretion of methotrexate.
ADVERSE EFFECTS :
Common : Diarrhea, nausea, vomiting which can be reduced by taking Augmorix in the start of meal ,
mucocutaneous candidiasis.
Uncommon : Dizziness , headache , skin rash , urticaria and moderate rise in AST and/or ALT.
Very rare : Crystalluria, anaphylaxis, antibiotic - associated colitis, hepatitis and cholestatic jaundice.
Convulsion which may occur in patients with impaired renal function or in those receiving
high doses.
OVERDOSE :
Symptoms and signs of overdose include gastrointestinal symptoms and disturbance of the fluid and
electrolyte balances which may be treated symptomatically. Augmorix can be removed from the circulation
by haemodialysis.
STORAGE INSTRUCTIONS :
Tablets : Store below 25oC, in a dry place , protect from light.
Dry suspension : Store below 25oC, in a dry place.
Suspension after reconstitution : Store in a refrigerator and use within 7 days.
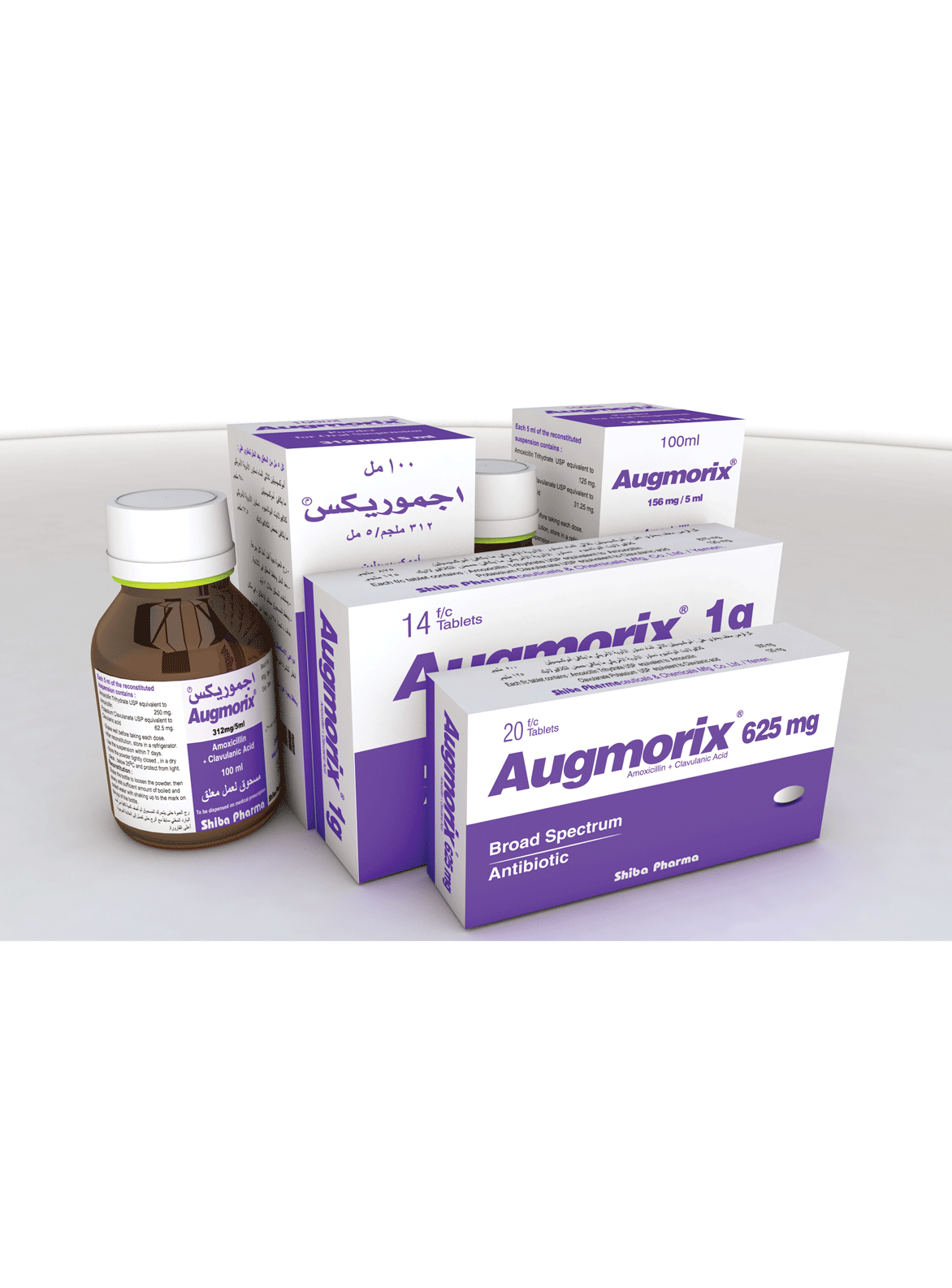
PRESENTATIONS :
Augmorix 625 mg Tablets : Pack of 20 tablets and hospital packs of different sizes.
Augmorix 1000 mg Tablets : Pack of 14 tablets and hospital packs of different sizes.
Augmorix 156 mg, 312 mg dry powder for Suspensions : Bottle of 100 ml (After reconstitution).
Augmorix
COMPOSITION :
Each F/C tablet of Augmorix 625 mg contains :
Amoxicillin Trihydrate USP equivalent to Amoxicillin 500 mg
Potassium Clavulanate USP equivalent to Clavulanic Acid 125 mg.
Each F/C tablet of Augmorix 1000 mg contains :
Amoxicillin Trihydrate USP equivalent to Amoxicillin 875 mg
Potassium Clavulanate USP equivalent to Clavulanic Acid 125 mg.
Each 5 ml of Augmorix 156.25 mg suspension (After reconstitution) contains :
Amoxicillin Trihydrate USP equivalent to Amoxicillin 125 mg
Potassium Clavulanate USP equivalent to Clavulanic Acid 31.25 mg.
Each 5 ml of Augmorix 312.5 mg suspension (After reconstitution) contains :
Amoxicillin Trihydrate USP equivalent to Amoxicillin 250 mg
Potassium Clavulanate USP equivalent to Clavulanic Acid 62.5 mg.
DESCRIPTION :
Augmorix (Amoxicillin and Clavulanic Acid) is a broad spectrum antibiotic with a bactericidal activity.
Its activity is due to the ß-lactamase inhibitory action of Clavulanate which extends the spectrum of
Amoxicillin to include wide range of organisms including Gram-positive, Gram-negative bacteria and many
bacteria resistant to other ß-lactams antibiotics. Absorption of Amoxicillin and Clavulanic Acid is optimised
when taken at the start of meal. After oral administration, Amoxicillin and Clavulanic Acid achieve
maximum plasma concentration in about one hour, both have a low levels of serum binding and about 70%
remains free in the serum. The main route of elimination is via urine. The half life of Amoxicillin and
Clavulanic Acid is approximately one hour.
INDICATIONS :
Augmorix is indicated for the following cases :
- Upper respiratory tract infections including ENT (e.g. tonsillitis , sinusitis and otitis media).
- Lower respiratory tract infections (e.g. acute and chronic bronchitis and bronchopneumonia).
- Genito-urinary tract (e.g. cystitis, urethritis and pyelonephritis) and abdominal infections.
- Skin and soft tissue infections (e.g. boils, abscesses, cellulitis and wound infections).
- Bone and joint infections (e.g. osteomyelitis).
- Dental infections (e.g. dentoalveolar abscess).
- Other infections (e.g. intra-abdominal sepsis).
DOSAGE AND ADMINISTRATION :
- The usual dosages are :
• Adults and children over 12 years :
- Mild - Moderate infections : One Augmorix 625 mg tablet twice daily .
- Severe infections : One Augmorix 1000 mg tablet twice daily.
- Dosage in dental infections : One Augmorix 625 mg tablet twice daily for 5 days.
- Augmorix 625 mg and Augmorix 1000 mg tablets are not recommended in children of 12 years and
under.
• Children :
- Under 1 Year : 25 mg/ kg / day.
- 1 - 6 years (10 - 18 kg) : 5 ml Augmorix 156.25 mg suspension three times a day.
- Over 6 years (18 - 40 kg) : 5 ml Augmorix 312.5 mg suspension three times a day.
- In more serious infections the dosage may be increased up to 50 mg / kg /day in divided doses
every 8 hours.
- Dosage in renal impairment :